Which of the Following Contains the Most Atoms
A 39098g K B 24304g Mg C 90122g Be D 2162g B Get the answers you need now. A100 g Ne B100 g He C100 g Ar D100 g Kr E100 g Mg By signing up youll get.

Solved Which Of The Following Contains The Most Atoms A 5 Chegg Com
Which of the following contains the MOST atoms.
. Thanks for your time in advance. The overall electrical charge of most atoms in balances because each atom contains an equal number of protons and electrons. B 100 g He.
1 mole of NH 3 4N A Atoms. D 100 g Kr. A 4g of H 2.
You shouldnt need to do a calculation here. 0 2 0 8 6. This is the best answer based on feedback and ratings.
Which of the following contains the most atoms. 64 g of SO 2 3 N A of atoms. How many atoms are present in 56 mg of Silicon.
Among the elements mentioned helium has the lowest molar mass. One mole of any substance is equal to the value of 6023 x 10 23 Avagadro numberIt can be used to measure the products obtained from the chemical reaction. 1 100 g Cs 100 g Mg 100 g Ca 100 g Rb 100 g Ne.
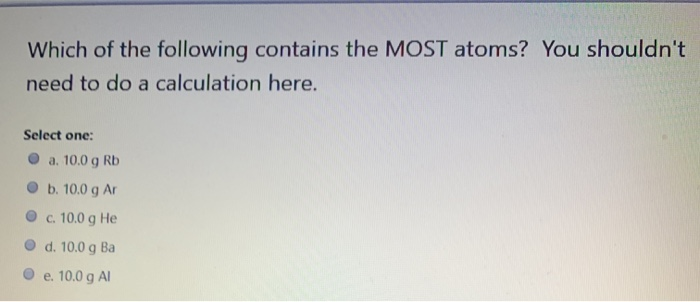
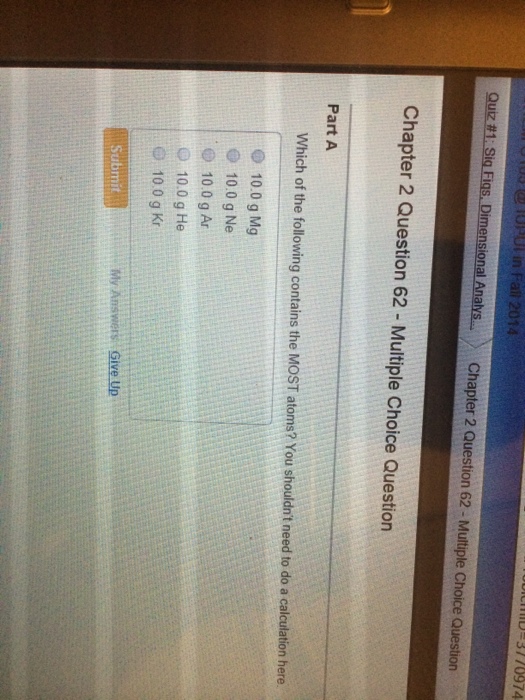
You shouldnt need to do a calculation here. Which of the following contains the MOST atoms. A 100 g Ne B 100 g He C 100 g Ar D 100 g Kr E 100 g Mg.
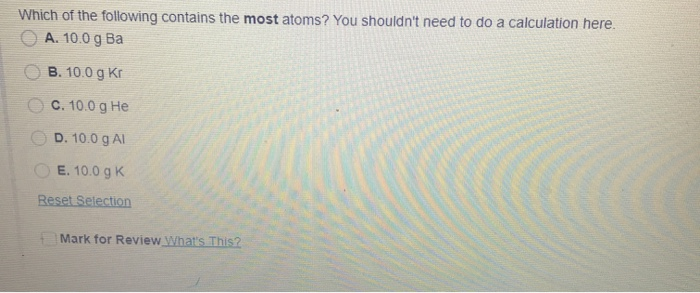
A 100 g Al B 100 g He C 100 g Ca D 100 g Kr E 100 g Cs. E 100 g Mg. Rank the following atoms in order of decreasing size ie largest to smallest.
4 g of hydrogen contains the largest number of atoms. Which of the following contains the MOST atoms. 39098 g K 2162 g B 90122 g Be 24305 g Mg.
7 6 1 0 2 2 atoms. You shouldnt need to do a calculation here. Therefore it will have highest number of moles and thus it.
Asked Apr 17 in Environmental Atmospheric Sciences by Smile92 oceanography. Rb F Mg B N. 1 mole of an element contains 6023 x 10 23 atoms.
0 2 3 1 0 2 3 3 3. Which of the following contains the most atoms. You shouldnt need to do a calculation here.
Which of the following contains the MOST atoms. I know the answer is B. E 100 g Ne Explaination Number of moles is given by.
You shouldnt have to do a calculation here A. So I have a homework question that states the following. Of atoms 0.
The unit is denoted by mol. C 100 g Ar. A 100 g Ne.
Answered Sep 20 2016 by. A mole fraction indicates the number of chemical elements. 24305g Mg 39098g K 90122g Be 2162g B Answered.
Hence all the three options have the same no. 8g of SO 2 38 N A of atoms. 17 g of NH 3 4N A atoms.
Asked Sep 20 2016 in Chemistry by Audra_Joy. 5 g of NH 3 2017 N A atoms. Therefore option D is correct.
A mole is defined as the mass of the substance which consists of an equal quantity of basic units. 1 mole of SO 2 3N A of Atoms. D 8g of SO 2.
You shouldnt need to do a calculation here. Up to 256 cash back Which of the following contains the MOST atoms. Which of the following contains the most atoms.
You shouldnt need to do a calculation here. How many atoms are present in 023 moles of Calcium. A 4 g of hydrogen molar mass 2 corresponds to 2 moles of molecules or 4N number of atoms where N is the Avogadros number 6.
Which of the following contains the most atoms. Now if we compare 4g of H 2 has more number of atoms. Which of the following contains the MOST atoms.
0 2 3 1 0 2 3. Which of the following contains the bartleby. Since number of moles mass of the element molar mass of the element.
Correct option is A a 05g atom mole of Cu05602310 23. Solution for Which of the following contains the most atoms. View the full answer.
Solved Which Of The Following Contains The Most Atoms You Chegg Com

Solved Which Of The Following Contains The Most Atoms You Chegg Com
Solved Which Of The Following Contains The Most Atoms You Chegg Com
Comments
Post a Comment